
 Dr. Toni Choueiri, the principal investigator, from Dana Farber Institute, presented the results of the much-anticipated Phase III trial, COSMIC-313, at the 2022 European Society for Medical Oncology (ESMO) Conference in Paris. COSMIC-313 is the first triplet drug trial in kidney cancer, in this case using cabozantinib (Cabometryx), nivolumab (Opdivo), and ipilimumab (Yervoy) versus the two-drug combination of nivolumab and iplimumab combined with a placebo in an 855-patient, first-line trial for patients with clear cell. The drugs were tested in only intermediate and poor risk patients, per International Metastatic RCC Database Consortium (IMDC)[1] criteria with 75% intermediate risk and 25% poor risk patients. Also, in both arms, only 65% of the patients had had a prior nephrectomy, which is unusually low.
Dr. Toni Choueiri, the principal investigator, from Dana Farber Institute, presented the results of the much-anticipated Phase III trial, COSMIC-313, at the 2022 European Society for Medical Oncology (ESMO) Conference in Paris. COSMIC-313 is the first triplet drug trial in kidney cancer, in this case using cabozantinib (Cabometryx), nivolumab (Opdivo), and ipilimumab (Yervoy) versus the two-drug combination of nivolumab and iplimumab combined with a placebo in an 855-patient, first-line trial for patients with clear cell. The drugs were tested in only intermediate and poor risk patients, per International Metastatic RCC Database Consortium (IMDC)[1] criteria with 75% intermediate risk and 25% poor risk patients. Also, in both arms, only 65% of the patients had had a prior nephrectomy, which is unusually low.
The manufacturer of cabozantinib is Exelixis, which sponsored the trial. The other components of the trial, nivolumab and ipilimumab, are manufactured by Bristol-Myers Squibb.
Background
This is the first time that a triple combination of anti-cancer drugs was tested in a trial for metastatic kidney cancer patients. The triplet consists of nivolumab (an immune checkpoint inhibitor that blocks PD-1), ipilimumab (a CTLA-4 inhibitor), and cabozantinib (a TKI that targets VEGF and MET).
The patients were limited to those with intermediate risk of dying (75% of the patients) and those with a poor risk for survival (25% of the patients). The intermediate and poor-risk patients were the categories where the combination nivo + ipi showed efficacy in a previous Phase III trial versus sunitinib.
The investigators expected the patients with a poor prognosis to have a higher response to the triplet than the intermediate-risk category patients. They also expected to see significant side effects from the triplet combination.
Results
At 12 months follow-up, the triplet arm patients had a 27% higher probability of not having their cancer progress versus the doublet arm (hazard ratio 0.73). The results were statistically significant.
The sub-group analysis showed that the Intermediate risk patients on the triplet combination had a 37% higher probability of not progressing (with median PFS not yet reached) versus those on the nivo and ipi combination (with median PFS of 11.4 months). The triplet arm had an Overall Response Rate (ORR) of 45% versus 35% for the nivo/ipi arm. However, the poor risk nivo/ipi, doublet group did better than the poor risk cabo/nivo/ipi, triplet group by 4%, with 11.2 months PFS versus 9.5 months PFS, respectively. The overall response rate was similar for the triplet versus the novo/ipi group at 37% versus 38%, respectively.
Two other subgroup statistics are of note. Patients under 65 years old who were on the triplet combination had a 32% higher probability of not progressing than those on the doublet nivo+ipi, however for those patients aged 65 and over, the probability drops to a 19% advantage. Secondly, those patients who did not have a prior nephrectomy before treatment and were prescribed the triplet combination had a 38% advantage in progression-free survival, but the PFS advantage dropped to 22% for the patients who had a prior nephrectomy.
The tumor response is shown in the following chart.
COSMIC-313 Tumor Response
Response Summary
This trial shows that the intermediate-risk patients did better on the triplet than they did on the doublet combination, at a 12-month follow-up, with a 37% higher probability of not progressing, with a PFS not yet reached versus 11.4 mos. on the doublet.
With respect to the poor-risk patients, the results favored use of the nivo+ipi therapy rather than the triplet cabo+nivo+ipi with a PFS of 11.2 mos. on the former combination versus 9.5 mos. for the latter. See graph below.
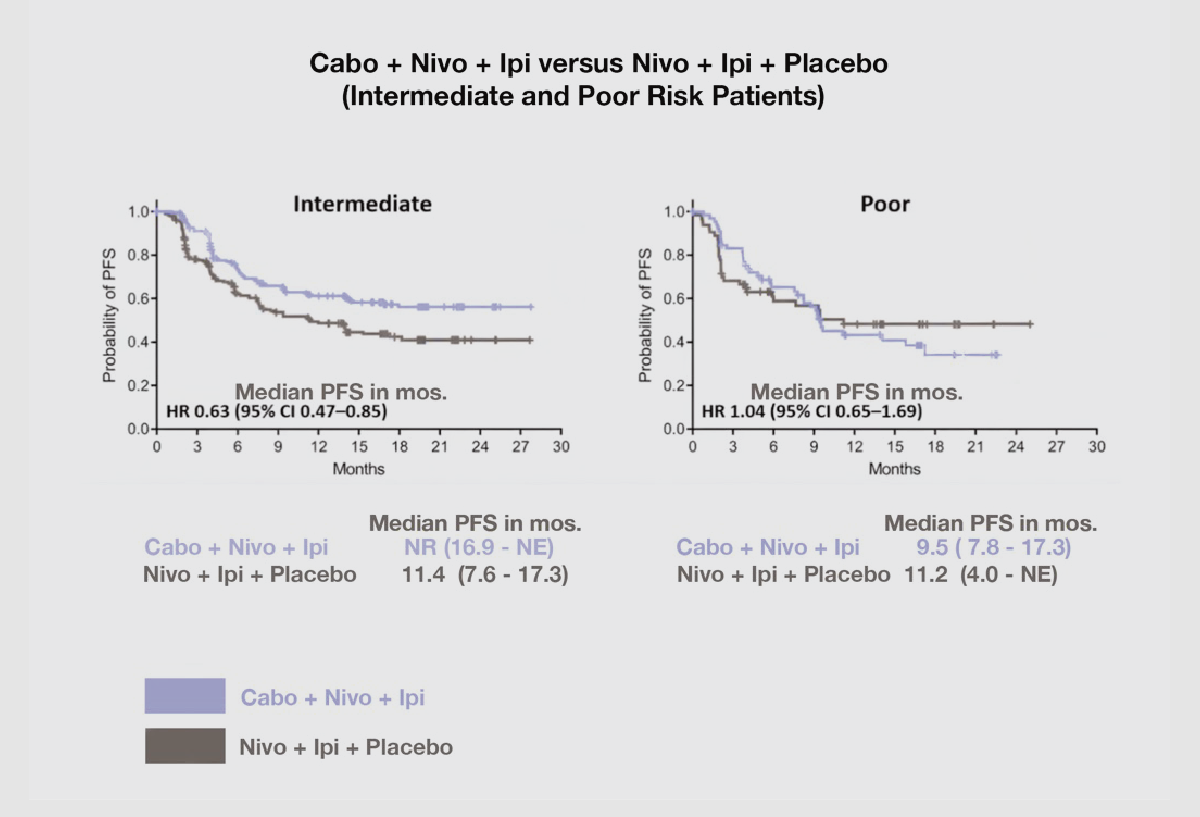
Source: Credit for Chart to Come
Safety Profile
One would expect the side effects to be worse for the triplet than for the doublet, which was true. Only 58% of the patients on the triplet completed all four prescribed ipilimumab doses versus 73% on the nivo+ipi arm did. 54% of the triplet therapy required a dose reduction in cabozantinib, and 12% of the triplet patients discontinued therapy altogether versus only 5% of the nivo+ipi arm patients.
The number of Adverse Events was higher in the triplet than the doublet by 54% versus 20%, including liver toxicity, diarrhea and skin issues. Corticosteroids were required in 58% of the triplet arm versus 35% in the nivo+ipi arm.
COSMIC-313 Safety
Discussant
 Dr. Samanta Pal from the City of Hope was the discussant. He was enthusiastic about the trial but said that they would have to wait for the overall survival data to make a final judgement. He added that the toxicity and quality of life issues must be dealt with. He and others found the results for the poor risk patients to be counterintuitive, and more work must be done to tease out the reasons for the worse performance of the triplet therapy used for poor risk patients.
Dr. Samanta Pal from the City of Hope was the discussant. He was enthusiastic about the trial but said that they would have to wait for the overall survival data to make a final judgement. He added that the toxicity and quality of life issues must be dealt with. He and others found the results for the poor risk patients to be counterintuitive, and more work must be done to tease out the reasons for the worse performance of the triplet therapy used for poor risk patients.
Dr. Samanta Pals’s chart is included below for reference. Note the hazard ratios of the cabo+nivo versus sunitinib for intermediate and poor risk categories as compared to those for the COSMIC-313 trial. For the intermediate risk patients, the hazard ratio was 0.58, or equivalent to a 42% lower probability of progressing on the cabo+nivo doublet than with sunitinib. This is similar to the triplet advantage in the COSMIC-313 trial. However, for poor risk patients, the cabo+nivo versus sunitinib trial yielded a hazard ratio of 0.36, or a 64% lower probability of progressing. The former numbers were what Choueiri and Pal were expecting, but didn’t get.
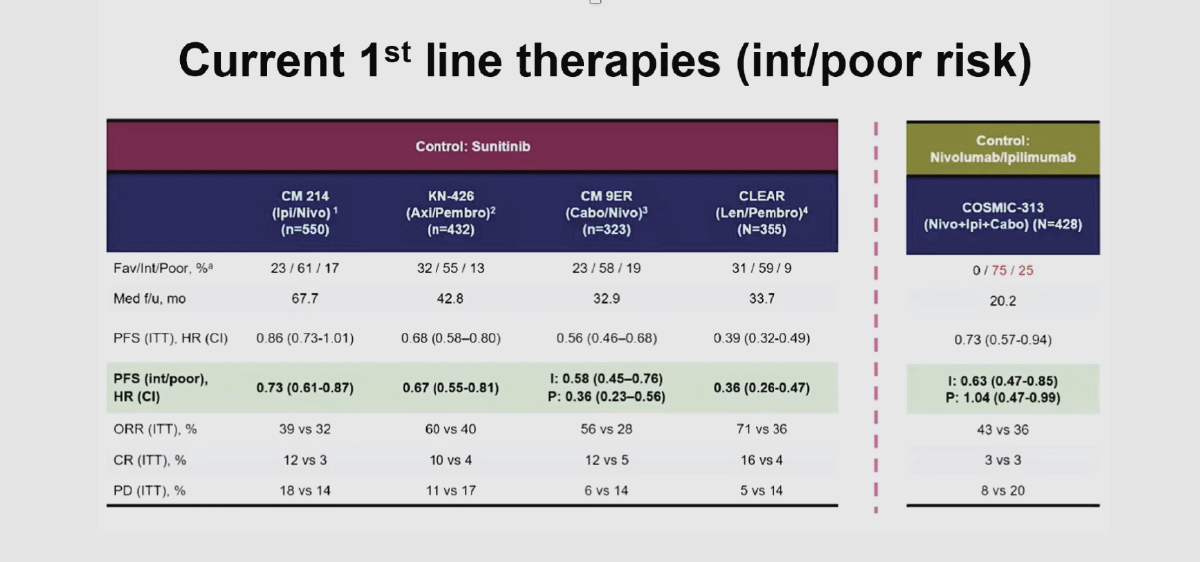
Source: Credit for Chart to Come

Fuel our fight to conquer kidney cancer! Engage with our blog and social media to help promote cancer awareness and spread the word in support of increased research funding. Join our 11K+ followers on Facebook.
We invite you to comment here!