Axitinib versus Sorafenib Phase III Trial
Brian Rini, from the Cleveland Clinic, presented the results of the Phase III AXIS Trial comparing axitinib versus sorafenib (Nexavar) as a second-line therapy. Axitinib is a tyrosine kinase inhibitor of the angiogenic receptors VEGFR1, VEGEFR21, and VEGEFR3. Axitinib has previously been tested in Phase II trials for cytokine-refractory[1] and sorafenib-refractory patients.The Time to Progression for axitinib for the cytokine-refractory trial was 15.7 months and for the sorafenib-refractory trial the PFS (progression-free survival) was 7.4 months. These are the first results for a trial that goes head-to-head with two targeted therapies, although a number are now being tested including sunitinib versus pazopanib.
The standard doses for the two therapies was used, 400 mg sorafenib, twice a day, and 5 mg axitinib, twice a day, with the option of titrating up to 10 mg if tolerated. The trial only accepted clear cell patients who had failed one prior therapy regimen including: sunitinib, temsirolimus, bevacizumab + interferon-alpha (INF), or a cytokine. Of the 723 patients randomized to one of the two arms, the vast majority failed either sunitinib (108) or a cytokine (70). Either 96% or 83% of the patients were classified a good or intermediate risk for survival, depending on which risk model you prefer, the MSKCC one or Heng’s.
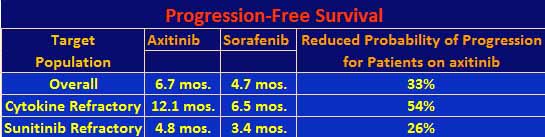
The results: axitinib patients had a median PFS of 6.7 months versus 4.7 months for sorafenib patients. The hazard ratio was 0.665, which means that the patients on the axitinib arm had a 33% less of a probability of progressing as compared to the sorafenib patients. The data were further stratified by prior regimen. Axitinib did much better than sorafenib for prior cytokine treatment with a PFS of 12.1 months as compared to 6.5 months, respectively, with a 54% lower probability of progressing. With respect to prior sunitinib treatment, the results were closer, 4.8 months versus 3.4 months, respectively, with a 26% lower chance of progressing. The investigators did a baseline characteristics analysis for PFS, with axitinib coming out better for most indicators including the fact that both women and men fared better on axitinib than on sorafenib, but women also did better than men. The PFS results are summarized in the following table. There were too few of the temsirolimus and bevacizumab refractory patients to make the results significant so they are not included. Note that overall survival data are not yet available for this trial.
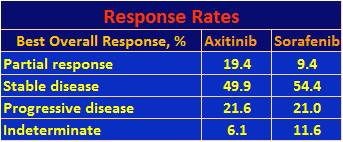
The overall response rates are given in the table below.
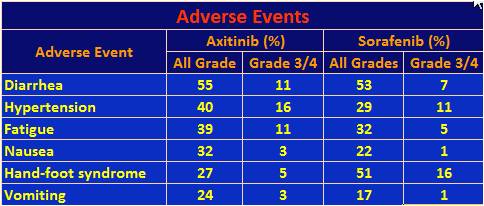
The following table lists adverse events that affected at least a quarter of the axitinib population.
37% of the axitinib patients had a dose increase, but another 31% required a dose decrease due to toxicity. By comparison, 54% of sorafenib patients required dose reductions. The results for these stratified populations will be presented at another time. Only 4% of axitinib patients discontinued treatment due to adverse events while 8% of sorafenib patients did so. So while the toxicities in the above table seem to favor sorafenib, they were not severe enough to cause as many dose reductions or discontinuance of treatment. Not included in the table were significantly higher rash and hair loss events for sorafenib. Also of note, in a separate presentation connected to this trial, other investigators looked at quality of life (QoL) as measured by patient provided questionnaires. The response rate to the survey was over 90%. It found that axitinib was no less tolerable than sorafenib.
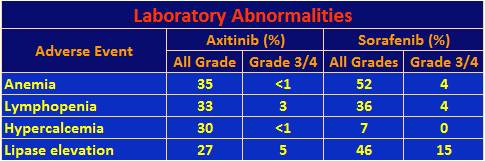
Laboratory abnormalities of high frequency were as follows.
Lymphopenia is defined as a decrease in white blood cells, which could lead to an increase in infection. Hypercalcemia is increased calcium in the blood, which can be accompanied by a host of symptoms from fatigue to loss of appetite. Increased lipase could lead to pancreatic issues.
In restrospective studies, it has been shown that there is a correlation between efficacy and hypertension. At last year’s ASCO, Eric Jonasch, from MD Anderson Cancer Center, announced a Phase II trial in which he will titrate (increase) the dosage of axitinib for patients who don’t exhibit hypertension to see if raising the blood pressure in these patients will increase the efficacy of the drug.
In summary, axitinib did significantly better than sorafenib, especially among cytokine-refractory patients but not so much with respect to sunitinib-refractory patients. All three drugs are tyrosine kinase inhibitors (TKIs). A commentator pointed out that everolimus, an mTOR inhibitor, demonstrated, in a Phase III trial, a PFS of 4.9 months for patients who had sometimes failed multiple prior therapies versus a single prior therapy in this trial. Of course, comparing numbers over multiple trials is not valid but only hypothesis generating, that is, there should be a second-line trial comparing several agents at once. Also, following a TKI by another TKI versus an mTOR inhibitor may produce different results.
On June 28th, the U.S. Food and Drug Administration (FDA) accepted Pfizer’s application for axitinib as a second-line therapy for renal cell carcinoma, based on the results of this trial. Earlier in the month, on June 1st, the European Medicines Agency (EMA), the European equivalent to the FDA, accepted Pfizer’s filing of axitinib for regulatory review, also for second-line therapy based on the results of this trial. Pfizer has always tested axitinib in a second-line setting, that is, with patients who were refractory to other therapies. This is understandable since Pfizer also makes the tyrosine kinase inhibitor sunitinib, which is the leading targeted therapy for first-line treatment of RCC. But once axitinib is approved, oncologists can prescribe it for first or second-line treatment. Let’s hope there will someday be biomarkers, as there are in breast cancer, that will inform them which therapy to try for which patient.
Finally, we take some personal satisfaction that axitinib will soon be approved for kidney cancer. In 2005, after a very favorable report on a Phase II study of the drug made at the ASCO Conference in San Francisco, Pfizer decided not to pursue development of the drug in kidney cancer, probably because they already had another drug further along in their pipeline, sunitinib. ACKC organized a letter writing campaign to Pfizer’s CEO requesting that the company develop the drug. In 2006, Pfizer reversed its position and planned a new trial for axitinib – they asked to meet with me in Atlanta at that summer’s ASCO Conference to inform me of their decision. We can only take partial credit for this result as the oncologist investigators and the developers themselves at Pfizer’s La Jolla research facility voiced their displeasure with Pfizer’s original decision.
[1] Cytokines for kidney cancer are Interleukin-2 and interferon-alpha (INF). Cytokine-refractory means that the patient failed a previous therapy with IL-2 or INF.