DoD Award for Enhancing Immune Checkpoint Inhibitors
by Jay Bitkower on October 19, 2015
Hans Hammers from Johns Hopkins Cancer Center won an Idea Award from the Department of Defense for Fiscal Year 2014 for “Enhancing Immune Checkpoint Inhibitor[1] Therapy in Kidney Cancer.” This is a two-year grant in the amount of $486,000 awarded to Dr. Hammers and Johns Hopkins. DoD grants have normally gone to pure researchers, but Dr. Hammers, aside from doing research, is also an eminent clinical oncologist who has made a number of presentations at ASCO (Association for Clinical Oncology) on his work in kidney cancer.
clinical oncologist who has made a number of presentations at ASCO (Association for Clinical Oncology) on his work in kidney cancer.
In the DoD study, Dr. Hammers will use local treatment of the tumor in an attempt to enhance the tumor response rate to immune checkpoint inhibitors. It is Dr.. Hammer’s theory that: “The objective response rate to immune checkpoint blockade in kidney cancer patients will be significantly improved by auto-vaccination approaches, i.e. release of antigen via radiation therapy or cryoablation or by the administration of pro-inflammatory TLR agonists[2]. We hypothesize that these interventions will lead to a systemic anti-tumor response, i.e. an “abscopal” effect and which can synergize with PD1 blockade”. In order to fully understand these concepts, we need some background.
In the last few years, with the development of nivolumab and other PD-1 and PDL-1 immune checkpoint inhibitors, immunotherapy has shown very encouraging results in clinical trials for the treatment of kidney, melanoma, and other cancers. As Dr. Hammers told us, “Immune checkpoint inhibitors are profoundly changing how we treat kidney cancer”.
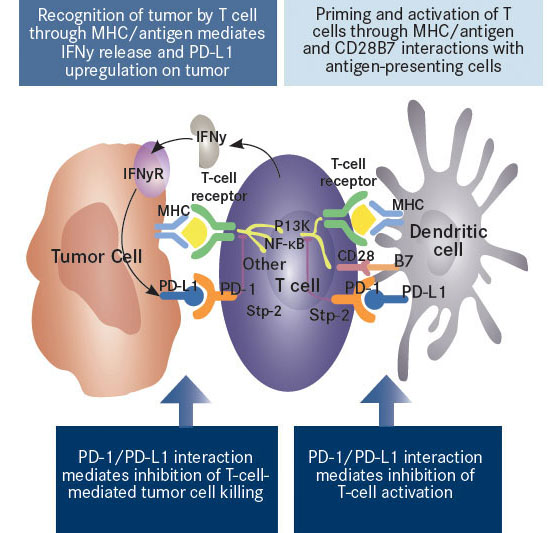
Nivolumab Mechanism of Action (before nivolumab inhibition)
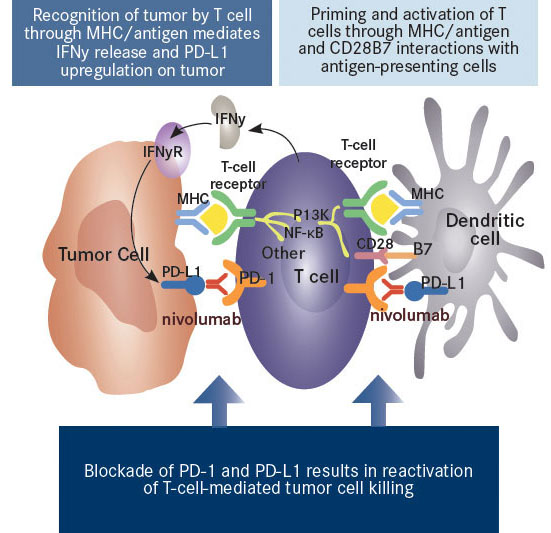
Nivolumab Mechanism of Action (after nivolumab inhibition – red antibody)
However, the response rate, as measured in clinical trials, to agents like nivolumab hovers around 20%, less than sunitinib with a response rate of 31% in the pivotal Phase 3 clinical trial. The promise for checkpoint inhibitors is in their potential. They’re like start-up companies that are currently losing money but which lure investors with the hope of super returns down the road. Why? Unlike targeted therapies, responders have experienced a durable, long-term response. Also, some checkpoint inhibitors have fewer side effects than targeted therapies, which augurs well for combining them with other agents. For example, when nivolumab was combined with another checkpoint inhibitor, ipilimumab, in a Phase I trial in kidney cancer, as Dr. Hammers reported at this year’s ASCO Meeting, the response rate jumped to 40%.
There are currently trials combining nivolumab with targeted therapies. But Dr. Hammers wants to go a step further. There has been some evidence, albeit rare, that when metastatic patients undergo stereotactic radiation or cryoablation (freezing) of their primary tumor, their secondary, or metastatic lesions, have decreased in size or even disappeared entirely. This may be due to the fact that, although the tumor itself is destroyed, the tumors’ proteins are still intact and are then recognized by the immune system as an antigen, something foreign to the body, and are targeted for destruction by activated T-cells.
Recognition of the tumor cells by the immune system is relevant since clinical trials using checkpoint inhibitors have demonstrated significantly higher response rates in patients who have an immune system that had a pre-established response to the tumor. In other words, those patients whose T-cells were already invading the tumor before receiving therapy have a higher response to checkpoint inhibitors than do those without any infiltration of the tumor by T-cells. This makes sense as we know that once the tumor is attacked by cytokines called interferon-gamma, which are emitted by the infiltrating T-cells, the tumor cells increase their PDL-1 receptors, which bind to the PD-1 receptors on the T-cells and disable them. The objective in using nivolumab and other PD-1 checkpoint inhibitors is to block the T-cells’ PD-1 receptors, and in doing so, prevent the tumor cells’ PDL-1 receptors from binding with PD-1, which would deactivate the T-cells. If there is no pre-established T-cell infiltration of the tumor then PD-1 inhibitors will be ineffective since the T-cells don’t recognize the tumor as being foreign in the first place so there is no inhibition of tumor destructive agents.
The phenomenon whereby local, or focal[3], treatment of cancer lesions by such methods as radiation and cryoablation causes a systemic effect reducing or even eliminating non-treated lesions throughout the body is called the abscopal effect. The cause of this effect, although not completely understood, is thought to be connected to the immune system’s recognition of the tumor cells as foreign, possibly by tumor-directed antibodies that increase at the same time that the abscopal effect occurs. The abscopal effect has been seen in only a few cancers, including kidney cancer and melanoma, which are, notably, the only two cancers that have responded to the immunotherapy High Dose Interleukin-2, an older form of immunotherapy, often associated with more side effects.
A newly recognized feature of the body’s innate immune system are TLRs, or toll-like receptors. A protein agent of TLRs has been shown to activate both the innate and adaptive immune systems to fight pathogens, including cancer cells. Dr. Hammers, in a pre-clinical setting, will alternatively use one of the TLR proteins called imiquimod[4] (which has been effective in destroying early-stage skin cancers), stereotactic radiation, or cryoablation to activate the immune system to recognize the tumor cells and combine each of these methods with the anti-PD-1 inhibitor nivolumab to theoretically increase the anti-tumor response in kidney cancer.
Per Dr. Hammers, the specific aims of the study are:
- To test the hypothesis that either high-dose localized radiation therapy or cryotherapy will augment the response to PD-1 blockade, resulting in improved local as well as distant disease control.
- To test the hypothesis that local administration of clinically relevant toll-like receptor (TLR) agonists will synergize with PD-1 blockade in mediating local and systemic anti-tumor immune responses.
- To optimize a combination treatment regimen for clinical translation, combining a local pro-inflammatory modality with TLR agonism and immune checkpoint blockade.
The DoD study will be conducted in a pre-clinical environment. However, in addition to the DoD study, Dr. Hammers is also planning to initiate a small trial of 25 kidney cancer patients, within the next six months, to test nivolumab/ipilimumab in combination with stereotactic radiation. He will start the patients on nivolumab and ipilimumab then will apply the stereotactic radiation in the hope that the response rate will rise above the 40% level found in the Phase 1 trial of the two drugs.
Importance of DoD Awards
We asked Dr. Hammers about the importance of Department of Defense’s Congressionally Directed Medically Directed Research Program
(CDMRP) to kidney cancer research. He responded citing two points. First, there is not much money for kidney cancer research, especially as compared to prostate cancer research, which has done well with its “mega-millions” (the five-year survival rate for prostate cancer is 99% versus 72% for kidney cancer patients). Therefore, DoD funding is critically important to allow kidney cancer researchers to move forward and to even remain in the field. Secondly, it is important to have a funding mechanism that encourages innovative ideas. To receive a grant from the NIH, for example, you often have to show research data to back up your hypothesis, in other words, you have to have completed some of your project in order to obtain the funding. DoD grants, on the other hand, accept higher risk projects that can offer higher rewards.
[1] Checkpoint inhibitors are part of the body’s immune system and function as a break against T-cell activity. For example, when one has an infection, T-cells rush to the site and destroy any pathogens that they find. But after the infection has abated, they may also start attacking normal cells. Checkpoint inhibitors are cell receptors that disable the T-cells when they are no longer needed to fight the infection. Unfortunately, many tumors have caught on to this phenomenon and grow their own checkpoint inhibitors to deactivate the T-cells that would ordinarily attack them.
[2] According to Wikipedia, “an agonist is a chemical that binds to a receptor and activates the receptor to produce a biological response”.
[3] Focal therapy is defined as treatment of individual lesions or small tumors, usually by non-invasive techniques. For example, focal treatment of a prostate cancer tumor would destroy the tumor but leave the rest of the prostate gland intact.
[4] Iiquimod is a TLR agonist. An agonist is a molecule that binds to a receptor on the surface of a cell and produces a response within the cell.