CARMENA Trial — Nephrectomy vs Systemic Therapy
At ASCO’s Annual Meeting in Chicago in June of this year, the major news in kidney cancer was the presentation of the results of the Carmena[1] Trial at a plenary session of the conference. This prospective, Phase III trial, which was run by French oncologists and reported on by Dr. Arnaud Méjean, from Hôpital Européen Georges Pompidou in Paris, showed that intermediate and high-risk patients who are diagnosed with metastatic cancer are as likely to benefit from medical treatment alone, rather than cytoreductive kidney surgery followed by medical treatment, in this case sunitinib (Sutent). This would be a reversal of the current established protocol, which is having the kidney removed first, followed by medical therapy for metastasis. In other words, it’s a big deal!
The treatment protocol for metastatic kidney cancer was established 15 years ago, before the era of targeted therapies, and consists of a nephrectomy followed by systemic medical therapy. The protocol continued with the introduction of targeted therapies such as sunitinib. That is now being challenged.
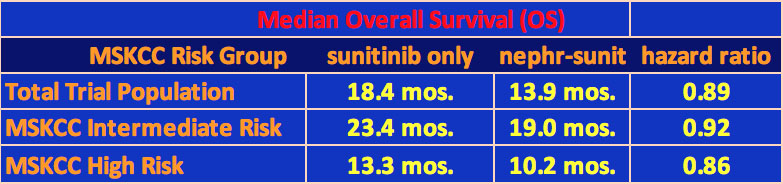
450 patients were enrolled in this trial from September 2009 to September 2017 with 326 deaths observed. The patients had clear cell pathology and all were metastatic. Approximately half the patients had nephrectomy followed by sunitinib while the other half had sunitinib alone. The standard dose of sunitinib was used in the study. The results of the trial as presented at ASCO and printed in an article in the June 3, 2018 New England Journal of Medicine (NEJM) “Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma”, stated that “The results in the sunitinib-alone group were noninferior to those in the nephrectomy–sunitinib group with regard to overall survival (stratified hazard ratio for death, 0.89” (a hazard ratio of 1 would imply no difference between the two while one equal to 0.5 would imply that the nephrectomy-sunitinib group would be twice as likely to die at any time)) – see NEJM article at https://www.nejm.org/doi/full/10.1056/NEJMoa1803675.
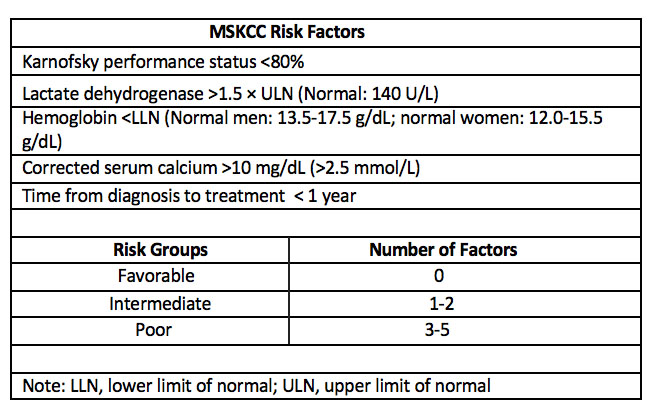
The median overall survival of the sunitinib only group was 18.4 months while that for the nephrectomy-sunitinib group was 13.9 months. The study was stratified by intermediate and high-risk patients using the Memorial Sloan-Kettering Cancer Center (MSKCC) risk factor for survival variables (see below for MSKCC risk factor categories). The survival results are shown in the table below.
There were no significant differences in response rate or progression-free survival for the patients in the two groups. In the sunitinib-only group, 38 of the patients underwent a secondary nephrectomy for emergency or other reasons. In addition, 11 patients in the group never received sunitinib. For the cytoreductive nephrectomy group, 95% of the patients had their entire kidney surgically removed.
The patients accepted in the trial had no previous therapy for their disease. The trial was conducted mostly at cancer centers in France but included some facilities in the UK, Sweden, and Norway.
Following Dr. Méjean’s presentation, Dr. Daniel George from the Duke Cancer Institute discussed the findings. Among other things, Dr. George also described two additional hypothesis for why cytoreductive nephrectomy may not have shown an overall survival benefit: “First, the primary tumor may serve as a source of neoantigens which may have benefit from an immunotherapy standpoint, and secondly, pre-clinical studies suggest that post-operative wound healing and inflammation may actually have a detrimental clinical effect and promote tumor growth”.
Based on the results of this trial, Dr. Méjean said that “cytoreductive nephrectomy should no longer be considered the standard of care in metastatic RCC”. But, not so fast. Certainly, the urologists in the U.S. would want to weigh in on this. Indeed, the NEJM also published a critique of the study by Drs. Paul Russo and Robert Motzer of Memorial Sloan-Kettering, see “Cytoreductive Nephrectomy — Patient Selection Is Key”:
https://www.nejm.org/doi/full/10.1056/NEJMe1806331?query=recirc_curatedRelated_article.
They said that since patient accrual for the trial occurred over several years, and, given that there were 79 centers involved, fewer than one patient was recruited per year per center. Russo/Motzer postulated that “surgeons saw patients with intermediate-risk disease who were likely to benefit from combination therapy, (so) they were unwilling for them to undergo randomization and instead treated them outside the trial”. Of course, this argument is speculation and there may be other reasons for the slow accrual, but it was slow.
Secondly, 43% of the patients were in the poor risk category, and the nephrectomy-sunitinib group had a higher percentage of Stage T3 and T4 tumors than the sunitinib-only group (by 70.1% to 51.0%). Patients with advanced disease are often not considered good candidates for surgery, one reason being that it would delay initiation of therapy to treat the metastasis. Russo/Motzer noted that MD Anderson Cancer Center, when they make their decision on whether to offer patients nephrectomies, uses stages T3 and T4 as one of their indicators that metastatic patients would not benefit from cytoreductive nephrectomies.
Finally, we are posting a video, provided to us by UT Southwestern, of a roundtable discussion of the trial with Dr. James Brugarolas, Chair of the Kidney Cancer Program at UT Southwestern, moderating, and including a UTSW urologist, Vitaly Margulis, and a UTSW oncologist, Hans Hammers. We encourage you to view the video, but are listing some of the salient points below.
- The trial is valuable in itself as heretofore there no prospective data available on targeted therapy and nephrectomy treatment options.
- In the U.S., poor risk patients are not often offered nephrectomies as they don’t live long enough to derive the benefits.
- There was a mismatch in randomization of patients between the two arms in that the nephrectomy-sunitinib arm had a larger percentage of patients with higher stage tumors and greater metastatic tumor burden, so that nephrectomies did not significantly reduce their overall tumor burden as much as was desired.
- There was a lot of crossover with patients on the sunitnib only group having subsequent nephrectomies, which could have affected the outcome.
- With the advent of cabozantinib and the newer immunotherapies such as nivolumab, the “old” targeted therapies such as sunitinib and pazopanib are no longer being considered as the go-to therapies in a first line setting.
A final comment was offered by Dr. Margulis, in response to a question from Dr. Brugarolas about this trial vis-à-vis a team approach. Dr. Margulis said: “This study highlights the importance of patient selection…with the importance of having a multidisciplinary team that assesses and evaluates the patient and comes up with a treatment plan … determining (in this case) which patients would benefit from systemic therapy only and which patients would benefit from a combined approach”. Dr. Hammers supported this comment and added the importance of the patient seeking out a major academic center, which sees a high volume of kidney cancer patients and which has better outcomes than at community centers, where kidney cancer may be a rarity. This is getting into infomercial territory, but they do have a point as it has been shown for example, that an experienced cancer practitioner can better get a patient through adverse events caused by a specific therapy than can a physician who has little experience with prescribing that therapy for their patients.
To see the video, go to Roundtable Discussion.
Final Comments
Dr. Méjean and his colleagues are to be commended for conducting a trial to determine, for intermediate and high risk, metastatic kidney cancer patients, whether the treatment protocol should be an initial nephrectomy followed by systemic therapy or systemic therapy alone. This is an important question from the patient’s perspective as the treatment protocol can affect survival just as a new medical therapy can. The investigators are trying to set a new standard for a 15-year old protocol, which, it seems, is not strictly adhered to anyway, especially by the large academic institutions that treat many kidney cancer patients. So, what is to be done?
- New trials should be developed to test whether cytoreductive nephrectomy followed by systemic therapy leads to longer overall survival than systemic therapy alone, or possibly even systemic therapy followed by nephrectomy, all stratified by risk category.
- If it’s important to develop risk variables for survival, then it is also important to develop variables similar to the ones used by MD Anderson for selecting nephrectomy candidates in order to inform the practitioner on how to make the optimal decision. The knowledge base that is developed from the broad experience of the large academic centers, should be codified so that the community practitioner who only infrequently treats metastatic patients can better serve their patients.
- Following from the above, organizations such as the American Urological Association (AUA) and the National Comprehensive Cancer Network (NCCN) should develop standards for cytoreductive treatment of metastatic kidney cancer for intermediate and high-risk patients. If currently there is insufficient information available to develop these standards, then they should design trials to settle the open questions.
[1] For those interested in name derivations, CARMENA is an acronym for Cancer du Rein Metastatique Nephrectomie et Antiangiogéniques





August 24, 2018 at 10:28 am, Sara Mears said:
Thank you ACKC for this thorough report!
September 25, 2018 at 2:15 pm, Patricia Robles said:
It would be nice if the oncologists and urologists established a standard protocol for treatment given the above trial results.