Combination Therapy Pembrolizumab/Axitinib Outperforms Sunitinib
In a report delivered by Thomas Powles, from Queen Mary University, London, the combination therapy of pembrolizumab (Keytruda) and axitinib (Inlyta) had a superior performance, in a 861-patient trial, to sunitinib (Sutent), the current standard of care therapy given for metastatic clear cell carcinoma (ccRCC). Based on the performance of pembrolizumab and axitinib in the trial, Keynote-426, the FDA approved the combination for first-line therapy for metastatic RCC.
The following report is based on Dr. Powell’s presentation at the ASCO Genitourinary Symposium February 2019, on the New England Journal of Medicine March 21, 2019 article “Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma,” and on the April 19 FDA approval announcement for the drug combination.
Both the anti-PD-1 pembrolizumab (immunotherapy) and the VEGFR tyrosine kinase inhibitor (TKI) have individually shown anti-tumor response as monotherapies in earlier renal RCC trials (these two trials were referenced by Dr. Powles at the beginning of his presentation):
- Pembrolizumab having a 38% overall response rate (ORR) with an 8.7-month progression-free survival (PFS) rate in a Phase 2 trial
- Axitinib having a 32% ORR and a 10.1 month PFS in a Phase 3 trial
Patient Characteristics
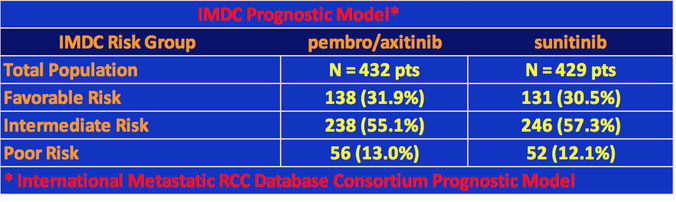
In this trial, patient were clear cell only with Stage IV disease and were treatment naïve (had no previous systemic therapy). Favorable, intermediate, and poor risk patients were all allowed in the study. The patients were divided into two arms with one arm given a combination of pembrolizumab and axitinib and the second arm, only sunitinib.
Some patient characteristics of the two groups were as follows. Over 70% were male and nearly half were from North America or Western Europe. About 18% of each arm had tumors with sarcomatoid features, and over 70% had two or more organs with metastases. The patient population’s risk categories prognostic for survival were:
Note: For an understanding of how Risk Categories are determined, please see IMDC on this website.
Pembrozilumab was administered intravenously at 200 mg once every three weeks. Axitinib was given orally at 5 mg twice a day, and sunitinib was given orally at the standard dose of 50 mg daily for four weeks followed by a two-week break. Both axitinib and sunitinib dosages could be varied depending on the patient’s tolerance of the drugs.
Discontinuance of Treatment and Survival Results
The median follow-up for analysis was 12.8 months, which was, for those who died, the time from first dose to the date of death, or for those still alive, the time from first dose to data cutoff (the total study period was 22 months).
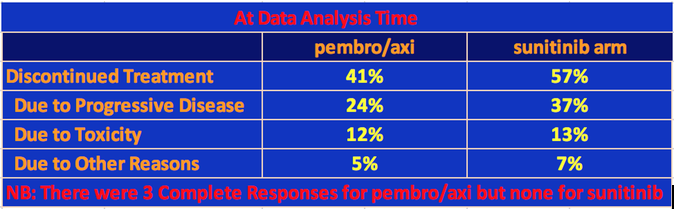
At data analysis, 41% of the patients had discontinued the pembro/axi arm of the trial and 56% had discontinued sunitinib. Most of the difference is due to the higher proclivity for tumor progression in the sunitinib population.
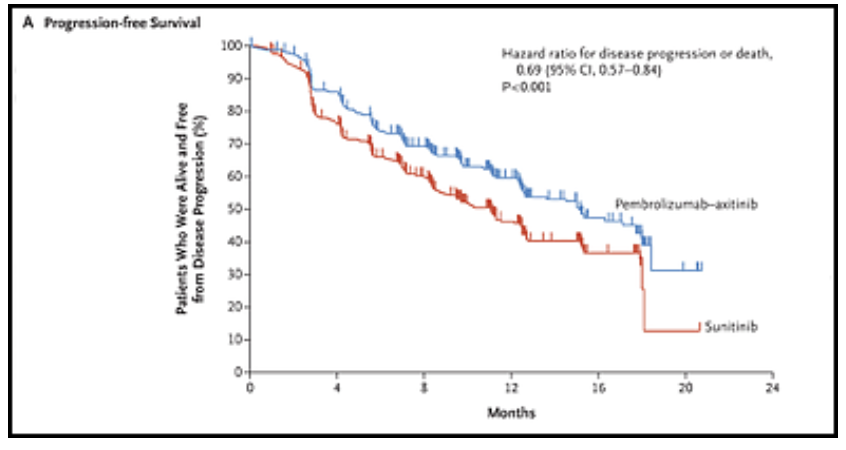
With respect to progression-free survival, the pembro/axi cohort had a median PFS equal to 15.1 mos. versus 11.1 mos. for sunitinib. All the PFS data are given in the following table.
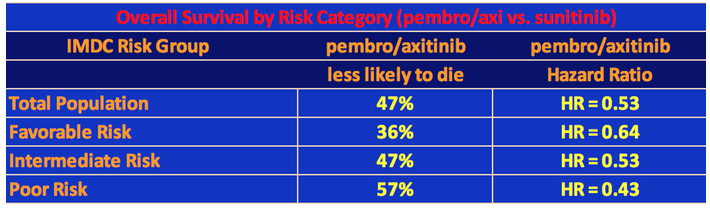
The 12-month overall survival rates were 89.9% and 78.3% for pembro/axi and sunitinib, respectively, which was highly significant statistically. At 18 months, the comparable rates were 82.3% versus 72.1%. All three risk group categories did better on pembro/axi than on sunitinib with the poorer the risk, the greater the survival of the pembro/axi patients versus the sunitinib patients for the same category. Note that researchers use the statistical term called hazard ratio to determine the likelihood of survival of one group over another. So, for example, patients in the Favorable Risk category who were taking pembro/axi were 36% less likely to die than the sunitinib patients in the Favorable Risk category. See the following table.
NB: For those interested in seeing how Hazard Ratio and the percentage of survival benefit of the test therapy versus thee control, see the following tutorial https://www.students4bestevidence.net/tutorial-hazard-ratios/). Secondly, these results are preliminary and neither treatment has yet reached overall survival so the statistics can change. Thirdly, these data refer to the trial population and not to an individual who is first put on therapy. It’s an average, broken down by risk category, which itself varies. Also, one’s predictive survival or response to a particular therapy is determined by many factors, most of which are unknown. Some patients have a very good response to sunitinib while others may not even be able to tolerate a specific side effect and stop taking the therapy, or they don’t have an oncologist who is confident or experienced enough to vary the dosage to ameliorate the side effects and keep the patient on the therapy. One of our constituents has survived over 12 years on sunitinib.
Finally, one should read “The Median Isn’t the Message” written by Stephen J. Gould, on our website at https://www.ackc.org/resources/stephen-j-gould-essay/.
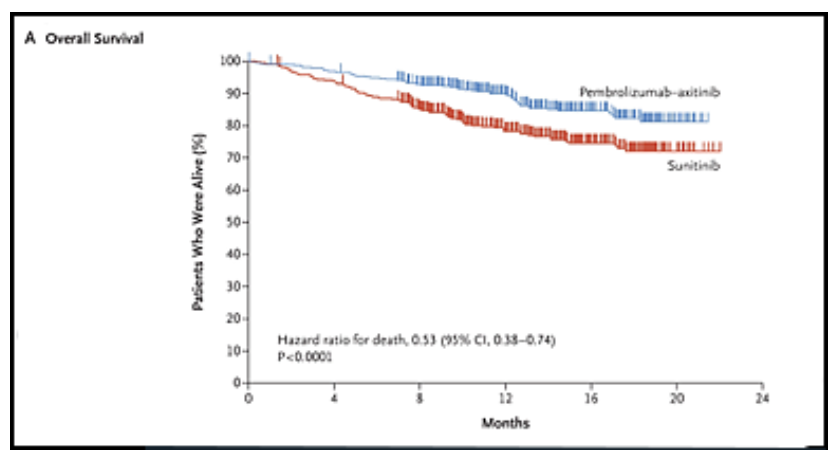
The survival curves for PFS and OS are given below.
Progression-Free Survival
Overall Survival
The two survival charts above are from a New England Journal of Medicine article, 2019; 380: 1116-1127 “Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma” by Brian Rini etal Copyright © 2019 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Median overall survival (OS) has not yet been reached for either pembro/axi or suntinib, but the HR = 0.53 indicated a 47% lower risk of dying with the combination therapy over sunitinib.
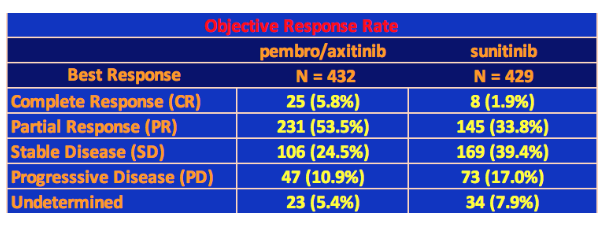
Overall Response Rate
Dr. Powles also presented the results for the overall response rate of the two arms of the trial, which is summarized below.
Note that these data were taken from the interim analysis taken in August 2018, the median follow-up time being 12.8 months (the time from randomization, or the first dosage, to death or the date of data cutoff for those who were still alive). Note that the study period was 22 months.
Unlike with TKI therapies, we are now accustomed to seeing complete responses with immunotherapy checkpoint inhibitors as well as long duration responses. The pembro/axi arm had 25 Complete Responses (CRs) amounting to 5.8% of the total patient population, which is similar to the response to High-Dose IL-2 trials in the past. Even sunitnib had 8 CRs. The 59% response rate for pembro/axi is high especially compared to the 36% rate for sunitinib. There were also fewer cases of progressive disease. It’s still too early to determine median overall survival, but with these results alone, it is clear that the combination of pembro/axi should replace sunitinib as the standard treatment for treatment naïve metastatic ccRCC (clear cell kidney cancer).
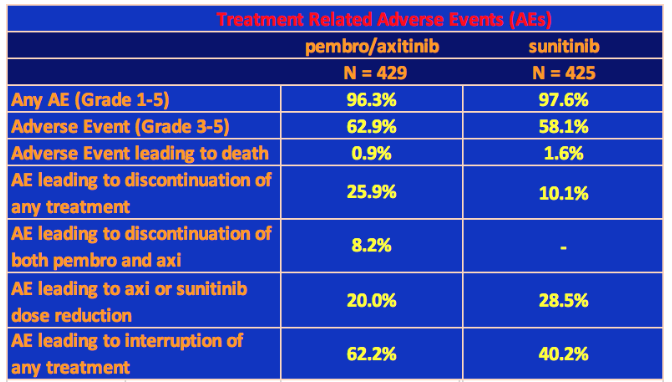
Adverse Events (Toxicity)
Let us take a look at the toxicity of the pembro-axi combination, or in clinical terms, the treatment-related Adverse Events.
Grade 3 or higher treatment-related adverse events of any cause occurred in 62.9% of patients in the pembrolizumab–axitinib group and in 58.1% in the sunitinib group. In the pembro/axi arm, Grade 3 or 4 hepatotoxicity (drug induced liver damage) occurred in 20% of patients resulting in discontinuation of pembrolizumab or axitinib in 13% of patients. The other significant treatment-related Grade 3-4 event was hypertension: 21% in pembro/axi arm vs. 18% in sunitinib arm.
In addition to hepatotoxicity and hypertension, the most common adverse events of any grade that occurred in 20% or more patients on the pembro/axi arm were: diarrhea, fatigue, hypothyroidism, decreased appetite, hand-foot syndrome, nausea, stomatitis, hoarseness, rash, cough, and constipation. Four people died on the pembro/axi arm due to treatment-related adverse events.
For the criteria used for Adverse Events Grades, see Grades on this website.
Summary
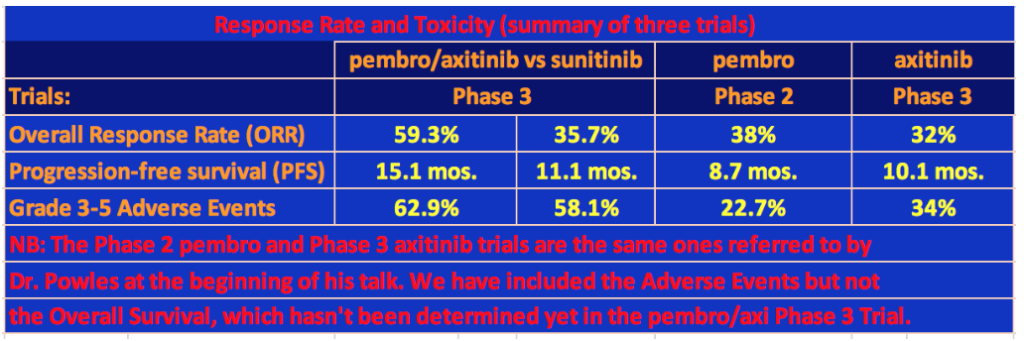
It’s clear that the pembro/axi arm of this trial outperformed sunitinib. The overall response rate (ORR) was 59.3% versus 35.7% for pembro/axi and sunitinib, respectively. The PFS was 15.1 mos. versus 11.1 mos. and there was a 47% advantage in overall survival for pembro/axi versus suntinib. In comparison, the ORR for the Phase 2 pembrolizumab monotherapy trial was 38% with a PFS of 8.7 months, and the ORR for the Phase 3 axitinib monotherapy trial was 32% and a PFS of 10.1 months.
However, this advantage does not come without cost. Grade 3-5 Adverse Events in the pembro/axi trial occurred in 62.9% of patients. In the same monotherapy trials that were mentioned by Dr. Powles at the beginning of his tpresentation, 22.7% of patients on pembrolizumab had Grade 3-5 Adverse Events (one patient died from the treatment) and 34% of the axitinib patients had Grade 3-5 Adverse Events (one person died of cardiac arrest). Finally, two and a half times more patients on the pembro/axi arm discontinued the trial due to toxicity than patients on the sunitinib arm.
Comments
Thomas Powles told this reporter that the results of the Phase 3 pembro/axi trial will establish the combination of pembrolizumab and axitinib as the standard of therapy for first-line therapy for clear cell metastatic renal cell carcinoma patients, competing with the combination of ipilimumab and nivolumab.
Others have made similar observations and have suggested the possibility of using ipi/nivo as a first-line treatment if the PD-L1 marker is high in a patient, otherwise using pembro/axi if VEGF is high, although VEGF has never prospectively been shown to be a marker for TKI response. In fact, in the 12 years from December 2005 through December 2017 when anti-VEGF targeted therapies held sway, the drug companies running the RCC trials never bothered to develop a biomarker to predict success of any of the dozen or so targeted therapies that were developed for ccRCC.
Now, in the immunotherapy (IO) era, where anti-PD-1 checkpoint drugs are predominant, even PD-L1 positivity has had mixed results, not always being a significant marker for successful treatment, while, at the same time, patients who are negative for PD-L1 can also have responses to this IO therapy. It is apparent that there are other things going on in the tumor microenvironment that the current science has not yet discovered.
In the last year, the industry has been developing various combination trials for RCC, for example, pairing an IO therapy like pembrolizumab with a TKI therapy like axitinib, or even combining two IO therapies like ipilimumab an nivolumab. There are other combinations already in trials, and this is a promising approach. But, as we have seen from the pembro/axi Phase 3 trial, a significant increase in performance has been accompanied by a big jump in toxicity. It remains to be seen if this toxicity will be tolerable in the long term for a significant number of patients. Further, it is still too early to tell about how many patients who are on IO therapy will have long-term, durable responses to this therapy. It is necessary to develop combinations of drugs that increase efficacy but reduce or, at least, stabilize the toxicity so that they are tolerable to a majority of patients.
Finally, monotherapies may still have a role in TCC treatment. For example, cabozantinib has demonstrated both anti-VEGF activity and efficacy against bone metastasis. Also, there have been a few long-term responders to sunitinib. It would be interesting to know why that is, and whether a predictive biomarker could be developed to see which patients might have a long-term response. This trial was also limited to clear cell patients. It is important to develop effective therapies for non-clear cell patients as well.