Comparative effectiveness of tumor response assessment methods: Standard-of-care versus computer-assisted response evaluation
Brian Allen, Assistant Professor of Radiology at Duke University, presented the following study.
Given the vascular nature of kidney cancer tumors, anti-angiogenic therapies such as sunitinib are prescribed to treat metastatic disease. The evaluation of the efficacy of the therapy is done via radiological exams that measure tumor size changes. However, the author claims that “tumor size changes are often delayed and underestimate tumor response in patients with metastatic RCC treated with anti-angiogenic targeted therapy”. New methods of evaluation use both tumor attenuation (density) and changes in tumor length to determine changes in the disease. So, even if a tumor has not decreased in size but has decreased attenuation or increased necrosis, it is judged to have responded well to the therapy.
Dr. Allen stated that most radiological evaluations are still done manually, e.g., measuring tumor size. He proposes using computer-assisted technology to reduce manual errors and automate calculations such as total tumor burden and change in tumor size from baseline. His group did a study of computer-assisted tumor response evaluation (CARE) methods versus manual analysis to determine if there is a reduction in errors and increase in interpretation speed using CARE. They evaluated 20 patients on the sunitinib arm of a Phase II trial. Eleven radiologists from 10 institutions participated in the study.
Common errors that they noted were selecting the incorrect target lesion, errors in size measurement, data transfer or mathematical errors, failure to identify marked decrease in attenuation, etc.
The results showed less than a less than 2% error rate in target selection and measurement for the manual evaluators versus a 0% for computer assisted evaluation, which identified errors in real-time, asking the evaluator to change his/her entries. Data transfer and calculation functions led to a 6% manual error rate versus 0% CARE method, which is automated. Finally, there was an up to 5% error rate, manual, in correctly categorizing the disease status as PR (partial response), SD (stable disease), or PD (progressive disease). With CARE, categorization is automated leading to a 0% error rate. Further, if the evaluation of tumor progression included evaluation of tumor attenuation and necrosis, the error rates increased with 30% of patient evaluations having at least one error. Finally, the mean evaluation time was twice as fast with CARE versus manual, 6.4 minutes versus 13.1 minutes for a tumor evaluation.
Dr. Allen noted some limitations of the study. It was a retrospective study, it underestimated the true error rate, and the study was not powered high enough to determine if and how much the errors would affect the distinction between tumor progression and stability, or whether therapy should be discontinued or not.
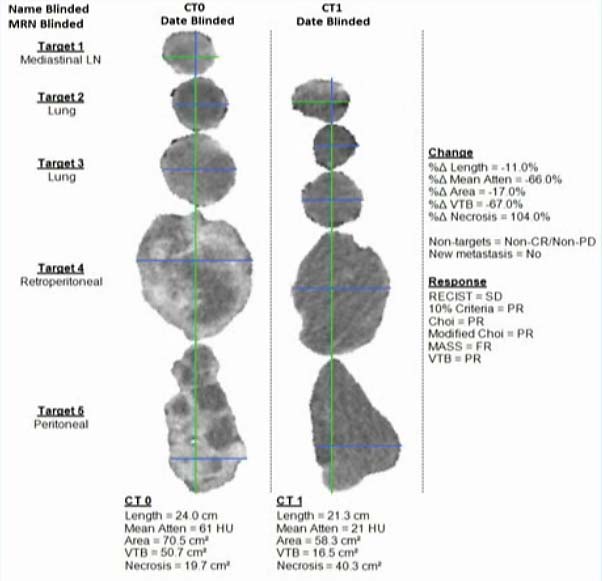
The following is a slide shown by Dr. Allen of a computer report that illustrates samples of target tumors before and after treatment. As can be seen, both the visual display and the accompanying statistics make clear that the therapy was working.
A prospective study of computer-assisted tumor response evaluation is needed, however it is clear that radiological analysis of tumor response to therapy will be automated as has been other professional work, leading to more accurate and faster evaluations and higher productivity.
This presentation was reviewed and commented on by Dr. Alessandro Volpe of the University of Eastern Piedmont in Novara, Italy. He welcomed the study and said that “the advantage of CARE may be even more significant in a real world scenario” since Dr. Allen’s study was carried out in large, heavy volume institutions that have experienced radiologists whereas smaller institutions can utilize a new tool to increase the efficiency and accuracy of radiological review of tumors. He also surmised that CARE could be used on 3-dimensional images and “dynamic contrast-enhanced images”. He also noted that evaluating efficacy of anti-angiogenic therapies will have to take into consideration of other factors than simply tumor size since the therapy can cause tumor necrosis even though the tumor size remains stable. Finally, given anti-angiogenic therapies, “tumor response assessment…is likely to undergo a substantial change in the next decade, with potential impact on clinical decision making and patient outcomes”. CARE is likely to be an integral part of that story.