Epacadostat plus pembrolizumab in patients with advanced RCC
The kidney cancer poster that garnered the most interest at the Association of Clinical Oncology (ASCO) annual meeting this summer was presented by Primo Lara of UC Davis showing the results of a Phase I/II trial using the combination of immune checkpoints epacadostat and pembrolizumab (Keytruda) in patients with advanced kidney cancer (NB: this combination is also being tested in breast and lung cancers and in other solid tumors, and pembrolizumab as a monotherapy has already been approved in other cancers but not in kidney cancer).
Rationale for the Study
Pembrolizumab, similar to nivolumab, is an inhibitor of PD-1 (an inhibitor of T-cells), and epacadostat is an inhibitor of the enzyme IDO (indoleamine 2,3-dioxygenase). So, what is IDO? The human body generates an inflammatory response in order to fight infection and injury. However, too much inflammation can damage tissues and even cause autoimmune disease, so the body also promotes the IDO enzyme, located in dendritic cells, to modulate the immune response and reduce inflammation. It seems that growing tumors have also learned this lesson, and they either express IDO on the surface of their cells or induce its expression in antigen presenting cells (dendritic cells and others). This phenomenon inhibits the activation of T-cells. In fact the “expression of IDO by tumor cells results in aggressive tumor growth and resistance to T-cell targeting immunotherapies”[1], and inhibition of IDO results in a reversal of immune suppression.
This Phase I/II trial recruited patients from several cancers with an expected enrollment of 463 patients but in only 33 in kidney cancer. Primo Lara reported on the results for RCC patients, all of whom had clear cell histology. The Phase II trial administered 100 mg of epacadostat twice a day and 200 mg of pembrolizumab once every three weeks. In all, the results for 27 patients were presented.
Side Effects
46 patients were evaluated for side effects. Of those, 80% had an AE (adverse event) with 16 AEs being Grade 3/4, although most of these were of single frequency. Two patients discontinued treatment due to AEs. The most common adverse events were fatigue, in 37% of patients (no Grade 3/4) and rash in 30% of patients (one Grade 3/4). Overall, the safety profile of the combination was similar to that in the Phase I trial in melanoma and other solid tumors. The incidence of rash was higher in the combination trial versus pemrolizumab as a monotherapy.
Response
Responders
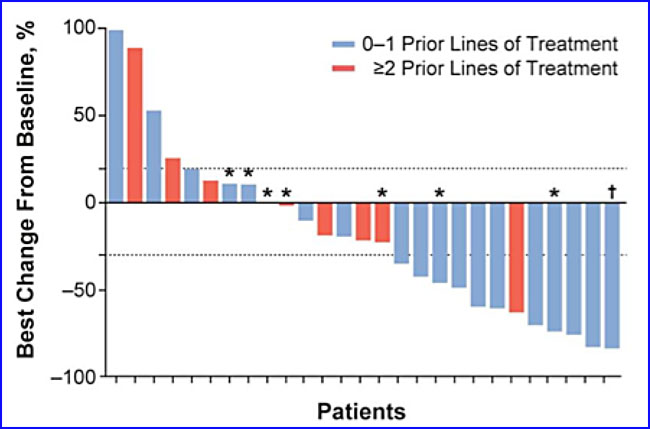
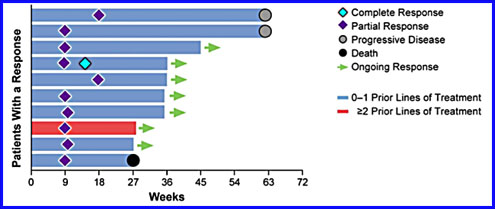
As can be seen from the graph, 12 of 27 patients (44%) had a PR (partial response) or a CR. Another ten patients (37%) had SD (stable disease). Seven patients eventually had their disease progress. The analysis for the responders to the treatment is given below.
Seven out of the ten responders have an ongoing response. There was no difference in response for patients who were positive for PD-L1 expression than for those who were negative. These statistics are enough for epacadostat’s manufacturer, a biopharmaceutical company called Incyte, to jointly schedule a Phase III trial for the combination with Merck, which will run the trial.
The Phase I/II trial is NCT02178722 on clinicaltrials.gov.
[1] Tumor-expressed IDO recruits and activates MDSCs in a Treg-dependent manner https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5013825/