Cabozantinib (XL-184) in Renal Cell Phase I Study
Ed. Note: The following report is from the ASCO 2012 General Meeting. There has not been a release of further trial data on cabozantinib since 2012, although there are now a Phase II and a Phase III trial for this therapy. See more on this and other comments at the end of this report.
Toni Choueiri of Dana Farber Cancer Institute presented a Phase I Study of the use of cabozantinib, a tyrosine kinase inhibitor (TKI), in clear cell renal patients and its interaction with another drug, Avandia. Cabozantinib is an inhibitor of both VEGFR2 and c-Met, both of which promote angiogenesis. The primary endpoints of the study were to evaluate the safety and tolerability and the efficacy of cabozantinib. Other institutions that participated in the trial included Brigham and Women’s Hospital and Beth Israel Deaconess in Boston, City of Hope in California, and St. Luke’s-Roosevelt in New York City.
25 patients were evaluated, all of whom had prior therapies for kidney cancer, mostly VEGF or mTOR inhibitors. This was a well-treated group as close to half of the patients had three or more prior therapies. For example, 14 of the 25 had previously taken sunitnib (Sutent). The dosage was 140 mg/daily, which was previously determined to be the maximum tolerated dose (MTD). Other studies have used 40 mg to 140 mg, with the lower dose showing efficacy in prostate cancer. Exelixis, the biotech company that developed the drug, will consider dose reduction in the future for rcc.
Response
The progression-free survival (pfs) for the 25 patients was 14.7 months. With respect to tumor shrinkage, 21 patients were evaluated, and, based on RECIST criteria, of those, 7 had a partial response (>30% shrinkage), 13 had stable disease, and one had progression. In total, 19 of the 21 evaluated patients had some shrinkage, although one had progressive disease due to new lesions.
Based on prostate trial data, cabozantinib has demonstrated significant efficacy in reducing or eliminating bone lesions. There were four rcc patients with bone lesions at baseline, two with pain. The pain resolved for both and one has continued to be pain-free for 73 weeks. One patient did develop new bone lesions. According to Exelixis, in addition to cabozantinib’s effect on osteoblasts and osteoclasts, an anti-tumor effect was also noticed in the bone lesions. It will be interesting to see further elucidation of this since, as compared with Zometa, which is an agent that has been used successfully to treat bone metastases, it is still unclear if there is an anti-tumor effect.
Side Effects
Adverse events (AEs) are listed in the following table. We included only those AEs that affected over 10% of the patients, except for hyponatremia, which had a high number of grade 3/4 responses. Note that hypophosphatemia is low level of phosphate in the blood, and hyponatremia refers to low levels of sodium).
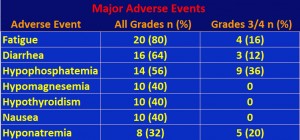
Interestingly, unlike other TKIs such as sunitnib and axitinib, there was only mild to moderate hypertension. There was some elevation in liver enzymes, but it was controllable.
Comments
What have we learned from this trial? First, this was a small Phase I trial, which usually focuses on side effects in response to giving the maximum tolerated dose. It is also difficult to compare this trial with advanced trials of the current rcc therapies given a different patient mix and much smaller patient recruitment for this trial. That being said, many of the patients on this trial had received extensive treatment for their disease prior to entry on the cabozanib trial. In our observation of trial results in general, second-line treatments (only one prior) have resulted in lower responses than with therapies in first-line, or treatment-naïve patients.
What stands out in this trial is the response rate, specifically the pfs of 14.7 months and some tumor shrinkage in 19 of the 21 evaluated patients for a patient mix that was heavily pre-treated. For example, of the 7 patients who showed partial tumor response (> 30% shrinkage), 4 of them had four or more prior treatments. The best pfs in current therapies is about a year, and this is mostly for treatment-naïve patients. The axitinib-sorafenib second-line trial (with one prior therapy) yielded a pfs of 6.7 months and 4.7 months for axitinib and sorafenib, respectively. Additionally, the patients on this trial were all in the intermediate and poor risk categories. Most trials have patients who have good and intermediate risk for recurrence. Cabozantinib also demonstrated efficacy against bone lesions, following its similar history with prostate cancer. The drug also seems to be well-tolerated without the significant hypertension and possibly concomitant heart issues found in other tyrosine kinase inhibitors.
Although the numbers are small, there doesn’t seem to be a relationship between number of prior therapies and response to cabozantinib. In other words, it doesn’t matter how many priors you’ve had, you still have a possibility of tumor response to cabozantinib – see table below.
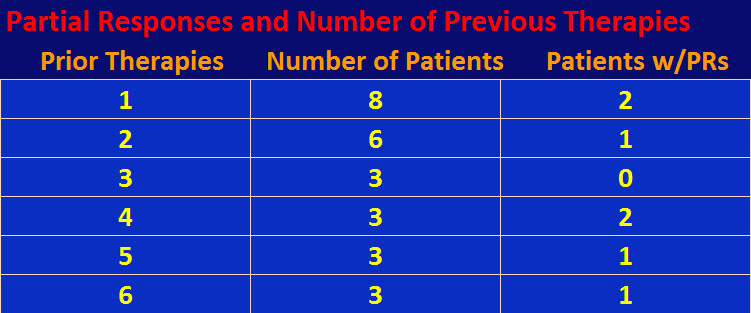
So what’s the downside? This is a small Phase I trial and these results must be confirmed by a larger Phase II trial. Exelixis, a small biotech company with limited resources, has been concentrating on cabozantinib in medullary thyroid and prostate cancer. Although, it is recruiting for a 600 to 1300-patient Phase II trial for solid tumors, not including rcc. Cabozantinib is a c-Met inhibitor, and silencing the c-Met gene can produce papillary renal cell carcinoma (prcc). Unfortunately, this trial explicitly called for clear cell rcc patients only (although one clear cell patient with papillary features did participate having stable disease). Any new trial should include prcc patients, especially given that foretinib, another c-Met inhibitor, has shown efficacy in prcc patients, and, secondly that none of the eight FDA-approved therapies for mrcc were approved for prcc.
Scientific Basis of Response
The targeted therapies developed to treat mrcc all have the objective of inhibiting angiogenesis, the proliferation of blood vessels that support metastatic tumor growth. The theory is that if you shut off the blood supply to the tumor, it will shrink and die. However, angiogenesis is only one of the hallmarks of cancer, see Hanahan and Weinberg, “Hallmarks of Cancer: The Next Generation” http://tinyurl.com/8ywaapm. Since these anti-angiogenic drugs eventually lose their effectiveness, there is much discussion of the mechanism of resistance. One idea is that when you inhibit the cell receptors of VEGF that promote angiogenesis, you create a low oxygen environment and the tumor cells split off and seek a more hospitable location. In other words, they metastasize. This mechanism has been observed in glioblastoma. The “splitting off’ seems to be a function of the scatter effect due to an up regulation of c-Met. Therefore, in order to increase effectiveness of an anti-cancer drug, you should inhibit both VEGF and c-Met. Doing so, you will attack a second hallmark of cancer, metastasis. Cabozantinib is an agent that has both features, and drug companies are developing their own similar agents to treat a variety of solid tumors or even combinations of VEGF and c-Met inhibitors. For example, the National Cancer Institute is sponsoring a trial, combining pazopanib, a VEGFR inhibitor, and ARQ 197, a c-Met inhibitor, in solid tumors.
Further Updated Comments
Cabozantinib is currently being tested in a Phase II trial (NCT01835158) comparing the drug against sunitinib (Sutent) in treatment-naive patients with advanced local or metastatic clear cell rcc (ccRCC). The target is 150 patients from the U.S. Recruitment is ongoing. One of the parameters that will be measured is the relation of high MET expression and response to therapy. It is also being tested in a Phase III trial (NCT01865747) of 650 patients comparing it against everolimus (Afinitor). The crieria include presence of clear cell pathology and having had at least one prior VEGF TKI therapy. The patient mix is worldwide, but recruitment is closed.
In a written report delivered at the 2014 ASCO conference, Michael Atkins and George Philips of Georgetown University mentioned cabozantinib as an agent that targets a new pathway, MET, in rcc. It’s necessary to attack new pathways so as to overcome resistance to the targeted therapies since, as they say “although overall survival … has improved substantially in the past decade the vast majority of patients still die of their disease”. In fact, there hasn’t been an increase in overall survival due to therapy since sunitinib was introduced in January 2006. Cabozantinib, which is being tested in several cancers and has been approved by the FDA for medullary thyroid cancer, has also demonstrated efficacy in bone metastases, which, along with high MET expression affect overall survival. It remains to be seen, from the results of the two trials, if the efficacy of cabozantinib will demonstrate a breakthrough in increased survival or will have only an incremental effect. The 2014 results in a prostate cancer trial did not show a statistically significant difference in overall survival for cabozantinib against prednisone even though it demonstrate much better progression-free survival statistics.
Updated February 2015.



